Advanced Therapies Congress & Expo 2021
The LSRNW attended our first virtual conference this year, joining the Advanced Therapies Congress and Expo 2021. The meeting ran over 3 days, and here’s a brief run down of the talks we attended:
Keynote Panel Discussion: State of the Industry
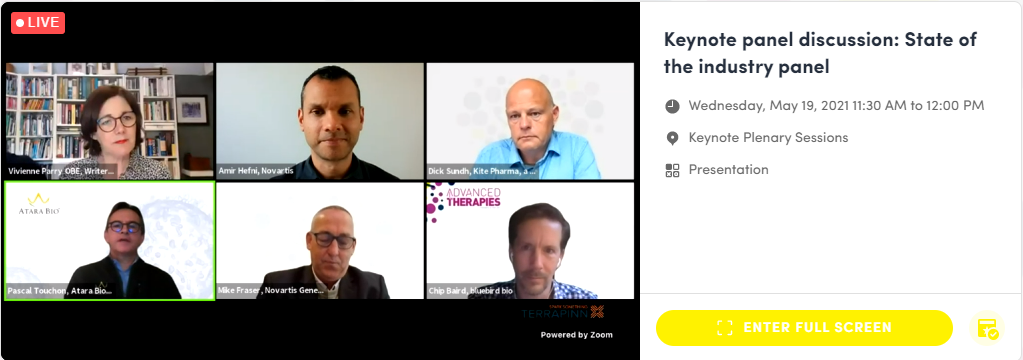
The discussion was chaired by Vivienne Parry, Head of Engagement at Genomics England, and had panelists from Novartis, Bluebird Bio, Atara Bio, and Kite Pharma, a Gilead company. The panel were extremely positive about the future for gene therapy, following the recent success seen in treating SMA-1. Regarding cell therapy, the panel were keen to point out that there is more to be excited about than just CAR-T cells and oncology, although this, in itself, remains a very positive area, with positive indications in the move to apply this technology to solid tumours. They highlighted the applications for cell-based therapies in autoimmune indications and for stem cell gene therapy. Future areas of growth include allogeneic cell therapy, and increased clinical application of CRISPR/Cas 9 gene editing.
Roundtable with the regulators: Ensuring clinical success

With 132 people joining the session, regulatory issues are clearly a hot topic in ATMP, as you might expect. With speakers from the FDA and the MHRA, there was lots to discuss, and lots of input from the delegates. Key questions were raised around the rolling reviews that the MHRA have implemented during the COVID pandemic; Janet Glassford, representing the MHRA, indicated that in future is likely that the MHRA will be returning to their standard procedures once the pandemic is over.
An interesting point was raised by the FDA’s Peter Marks around viral vectors, who suggested a future move away from viral vectors, pointing out that while AAV vectors are (correctly) designated as non-integrating, at very high doses some genomic integration has been observed. Glassford (MHRA) suggested that the real-world success of mRNA vaccines might see a move towards direct use of nucleic acids, without the need for viral vectors at all.
The final note came from Debra Miller, CEO of CureDuchenne, who, I think, spoke for all rare disease patients and advocates when she said she would like to see the sense of urgency that was generated by the pandemic maintained – the fatality rate for most rare diseases is 100%, and these patients don’t have time on their side.
Gamma delta T cells: Potential off the shelf treatment for solid tumours – John Maher
An entirely different tone was struck by the talk delivered by John Maher, “Gamma delta T cells: Potential off the shelf treatment for solid tumours”. While he was there mainly in his capacity as CSO for Leucid, Maher’s talk felt very academic, with lots of nice data regarding his studies into the expansion of gamma delta T cells using IL2 and TGF beta. Of particular interest to me was the finding that these cells upregulated CXCR4, suggesting improved homing. I’m not sure whether I misunderstood, however, when he also mentioned them upregulating E-selectin – sadly my notes shed no further light on whether he was actually referring to E-selectin ligands, or whether he did indeed find E-selectin expressed on T cells, in contrast to their usual endothelial expression. If anyone else has better notes – let me know! Mysteries around E-selectin aside, interested preclinical data were presented that aligned with the hypothesis that these cells had improved homing, particularly to the bone marrow, and that they also produced more cytotoxic cytokines.
Roundtable: All the things you were too afraid to ask about funding a new technology or financing a new company

On the final day, some interesting tips were available from experts representing VC and investment companies, in the Roundtable “All the things you were too afraid to ask about funding a new technology or financing a new company”. Speakers form Antion Biosciences, Seroba Life Sciences, Syncona Investment Management Ltd and 4BIO Capital had the following advice:
- Make sure your pitch demonstrates your passion and motivation.
- View your relationship with your VC partners as long-term, and make sure they’re people you’re happy to work with! Make sure your visions align, and that everybody’s timescales and expectations are realistic.
- Make sure your company valuation is grounded in reality, although they do understand that early-stage tech is hard to value.
- Solid pre-clinical safety data is very positive – and you should ensure this is using the best model, not just the one you have to hand. If several models are needed, do this, and explain why. CROs/CDMOs can be useful partners for this stage, and VCs are likely to give additional credibility to their results
- If there’s a standard of care that you can compare your innovation to, that is really useful.
- Be honest – if you haven’t done something that is key to the preclinical data due to cost, say this. It shows that you know what you’re doing, even if you haven’t been able to do it yet.
Attracting, Retaining and Training Talent
The final session I attended was focused on Attracting, Retaining and Training Talent, an area close to our hearts at LSRNW. It’s estimated that by 2030, the UK will need 130,000 skilled workers in this area in order to deliver on our goals for ATMPs. The talk was very London-centric, with representatives from Imperial College, KCL and London Advanced Therapies. Given the existence of the Midlands and Wales Advanced Therapy Treatment Centre, I felt there was a lot that Wales could learn about developing local expertise in this area. Of particular note was KCL’s Stem Cell and Regenerative Therapies: From Bench to Market MSc course, as well as their Biological and Advanced Therapies short courses. There were some interesting viewpoints from Yvette Cleland (CPL Life Sciences) who talked about how apprenticeships are underused as a method of upskilling the existing workforce. Indeed, any extensive discussion about the “leaky pipeline” and the issue of maintaining a skilled life sciences workforce post-PhD was missing from this otherwise in-depth session.
If you’re doing research in this area, make sure to book your place at our Advanced Therapies Special Event on June 17th 2021.

